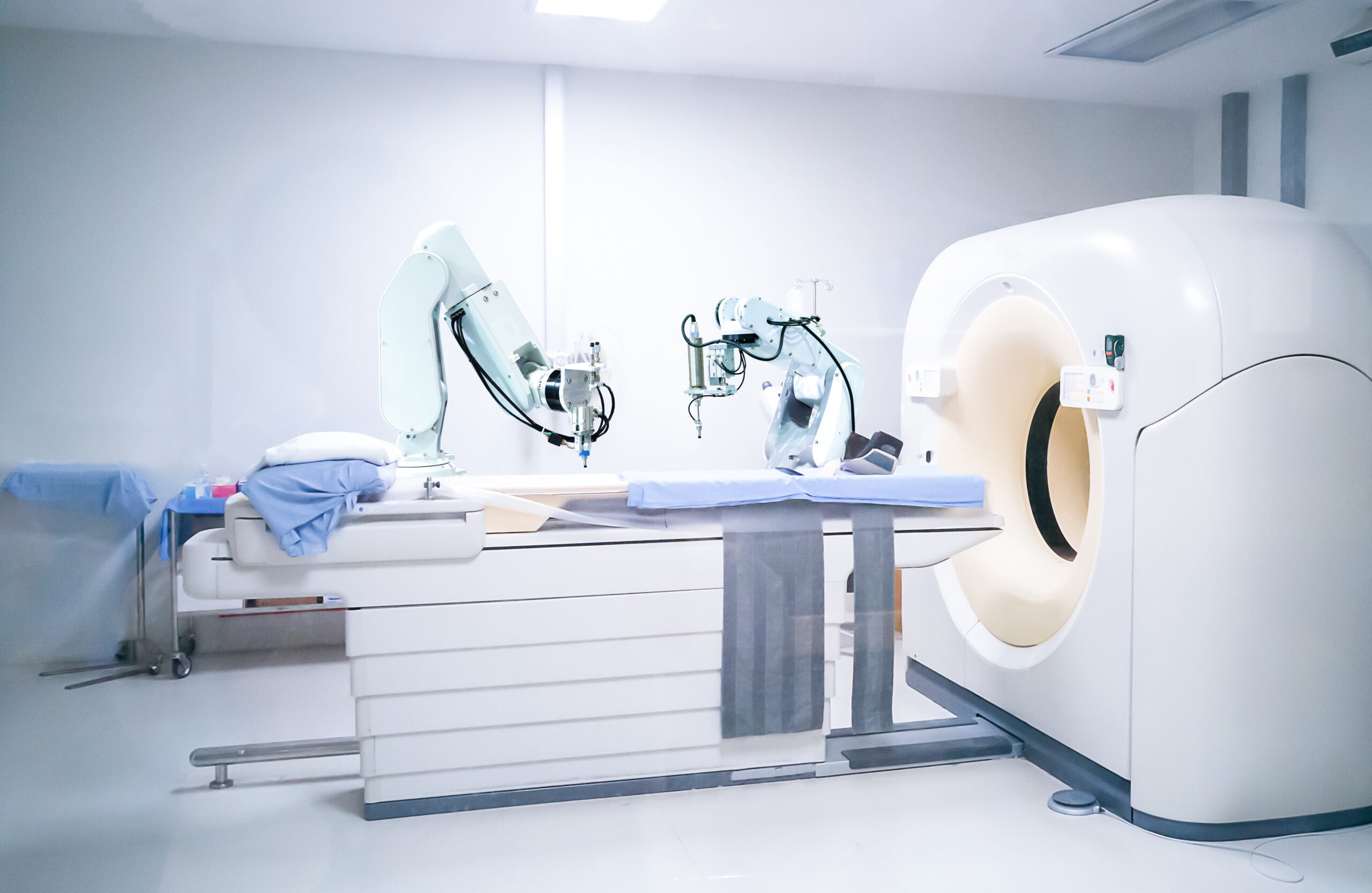
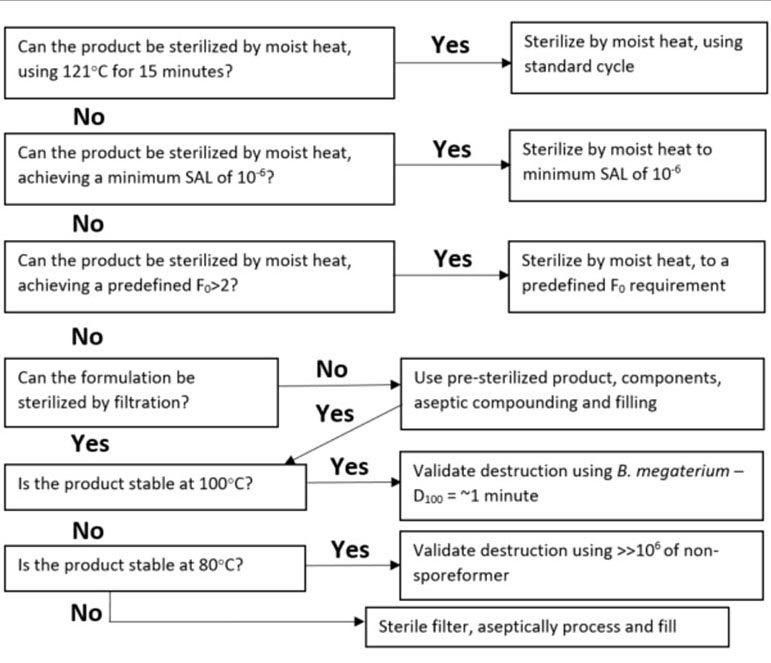
FDA Letter: Use Correct Cycle When Decontaminating Respirators with STERRAD Sterilization Systems - OR Today

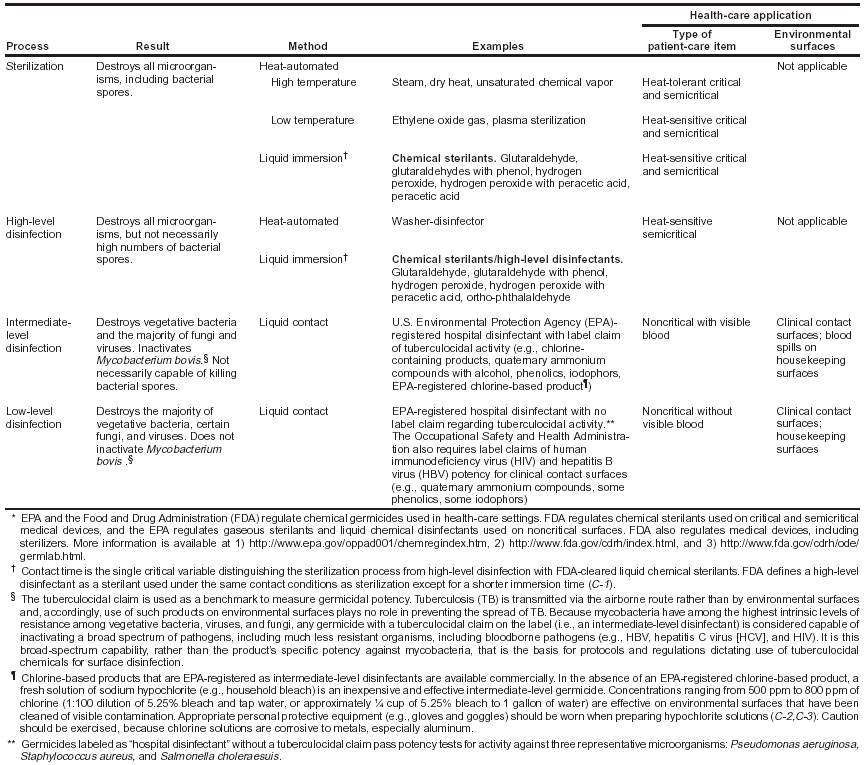
Comparison of the sporicidal activity of a UV-C High Level disinfection process with three FDA Chemical sterilants - Germitec Global

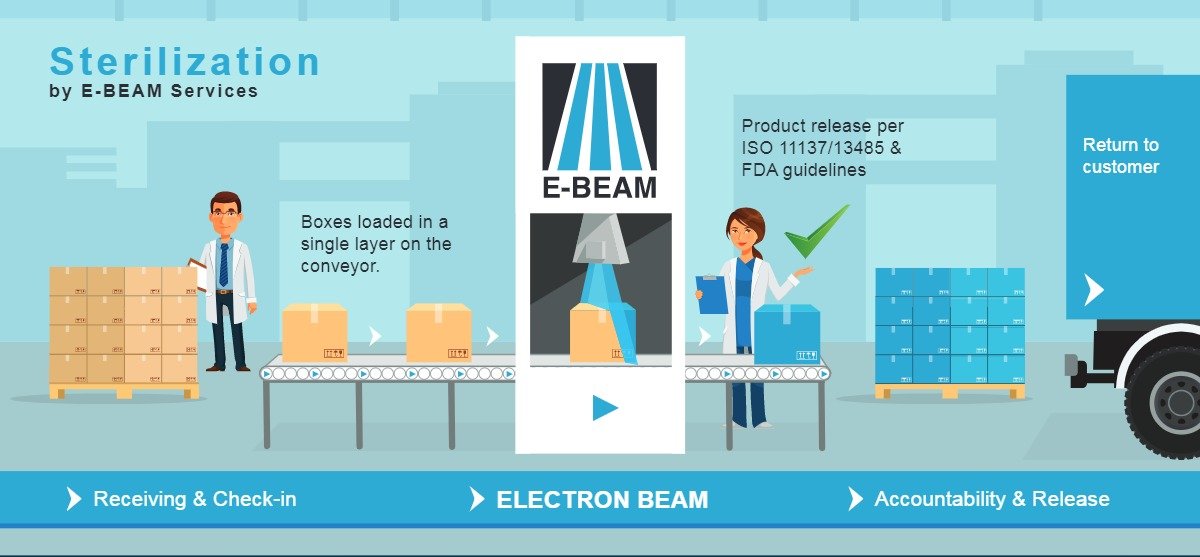
FDA Issues Predetermined Change Control Plan for AI/ML-Enabled Device Draft Guidance and EPA proposes rules on EtO medical device sterilization - Simbex